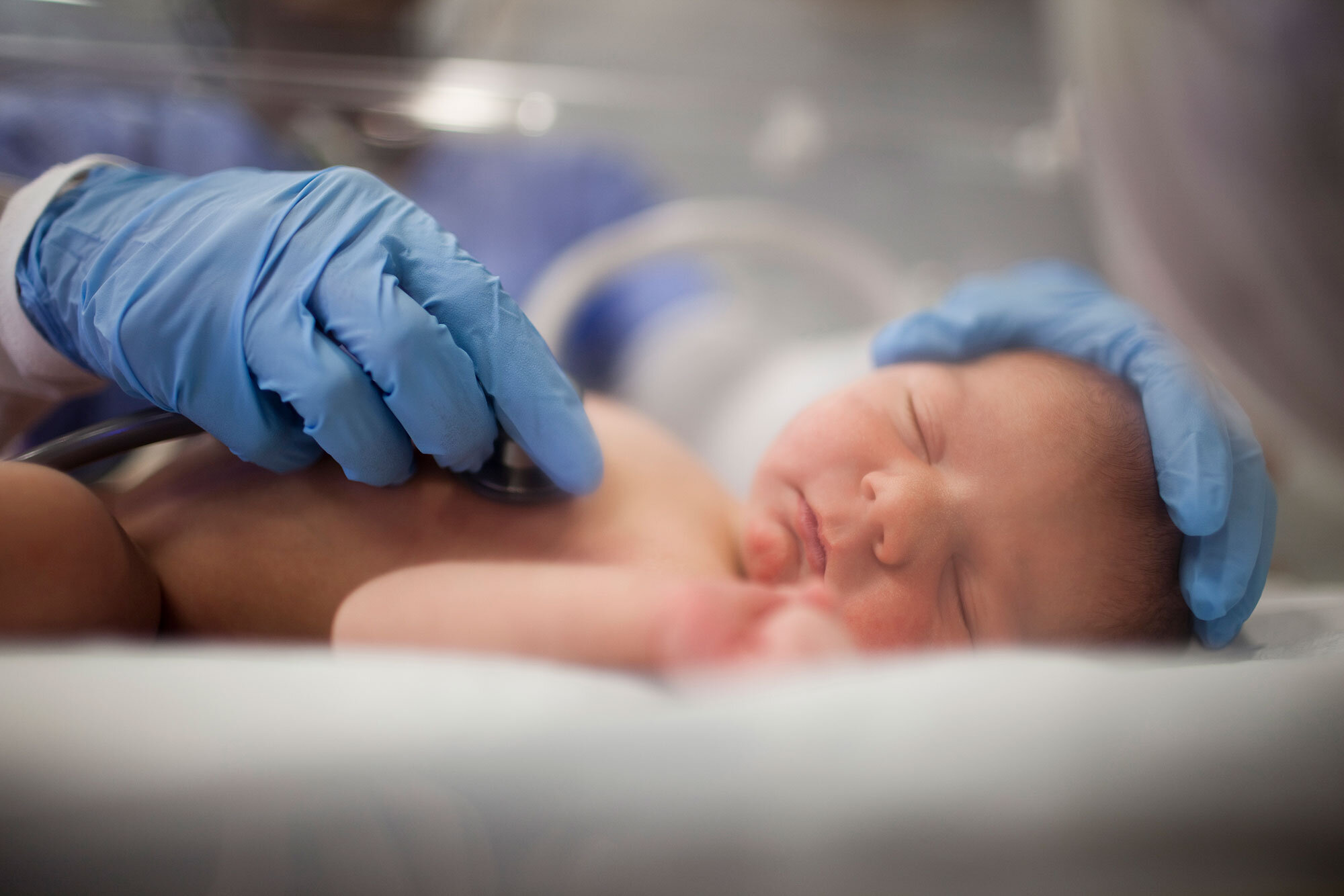
What products are sterilized at the Sterigenics Atlanta facility?
When operating at normal capacity, the facility sterilizes more than one million medical products and devices each day.
This includes surgical instruments, catheters and procedure kits, IV administration sets, personal protective equipment (PPE) such as drapes/gowns, wound protection sleeves, tubing sets and many more.
Without these essential devices, vital procedures would not occur and patients’ lives would be at risk.
Why is EO sterilization necessary?
As explained by the FDA, for many medical devices, sterilization with EO is the only method that effectively sterilizes and does not damage the device during the sterilization process.
EO is the only sterilization method that satisfies FDA-approved sterility validations for many critical medical devices.
Approximately 50% of sterile medical products in the U.S. are sterilized using EO.
Why can’t Sterigenics use other forms of sterilization instead of EO?
In late 2019, the FDA stated, “there are no readily available processes or facilities that can serve as viable alternatives to those that use ethylene oxide to sterilize these devices. In short: this method is critical to our health care system and to the continued availability of safe, effective and high-quality medical devices.”
Sterigenics provides sterilization services using gamma, EO, E-beam and X-ray technologies. The Atlanta facility only uses EO as required for the products sterilized at the facility, and has for nearly 50 years.
Sterigenics does not determine which sterilization method to use. The method of sterilization is determined by FDA sterility requirements and the medical product manufacturer based on the materials and the design of the product being sterilized.
If the Atlanta facility is safe, why did Sterigenics make enhancements to its facility?
Sterigenics installed additional enhancements to further reduce EO emissions beyond already safe levels.
The enhancements establish the Atlanta facility as one of the most advanced sterilization facilities in Georgia and the United States in terms of emission control.
How does the Sterigenics Atlanta facility compare to other sterilization facilities in Georgia and around the United States?
The recently installed enhancements establish the Atlanta facility as one of the most advanced sterilization facilities in Georgia and the United States in terms of emission control.
The Sterigenics Atlanta facility captures and controls 100% of the total EO used, including process emissions and fugitive emissions, outperforming regulatory requirements.
Is it safe to be at or near an EO facility?
It is safe to be at or near the facility. Sterigenics does not pose a risk to public health.
Should I be concerned that I am exposed to EO?
EO is in the air around us — in Georgia and across the country — but not at harmful levels.
EO is a common building block in many of the products we use on a daily basis, including household cleaners, personal care products and pharmaceuticals. It is also emitted from car and truck engines and natural gas combustion, and is produced naturally by plants and the human body.
The Georgia Department of Public Health reviewed the zip codes adjacent to Sterigenics’ facility and did not find “any evidence of increased incidents of cancers… that have been known to be associated with ethylene oxide.”
Is Sterigenics in compliance with all relevant rules and regulations?
Yes. The Sterigenics Atlanta facility has been industry leading, consistently outperforming regulatory standards for decades.
The facility has provided EO sterilization services for nearly 50 years. During that time, Cobb County has consistently certified that the Sterigenics facility complied with applicable County fire and building codes in connection with approved expansions and developments.
Why did Sterigenics file a lawsuit against Cobb County?
The County officials’ unlawful actions left Sterigenics with no choice but to take legal action in order to resume safe and vital operations at our facility.
Sterigenics worked in good faith to try to resolve the County officials’ unfounded certificate of occupancy claims for six months.
Unfortunately, legal action is necessary to protect Sterigenics’ rights and allow the facility to operate to meet the urgent needs facing health care workers and patients.
Why is Sterigenics allowed to operate today?
Following Sterigenics’ filing of its lawsuit against Cobb County, the United States District Court for the Northern District of Georgia entered a Temporary Restraining Order on April 1 temporarily prohibiting the County officials’ preclusion of the facility’s operations and ordering that Sterigenics be free to conduct its normal operations at the facility to sterilize medical products without the County officials’ interference.
On April 8, the Court entered a Consent Order entitling Sterigenics to continue its normal sterilization operations at the facility during the litigation and until the Court enters a final judgment in the case.
How many facilities does Sterigenics operate in Georgia? Does Sterigenics have any off-site storage warehouses?
Sterigenics operates one facility in Georgia. The company sterilizes vital medical products and devices at that facility.
The company does not lease or operate any other warehouses or facilities in Georgia.
What is Sterigenics’ response to the lawsuit filed by workers related to their work with products sterilized using EO?
The plaintiffs in this lawsuit are a customer’s employees – they are not employed by Sterigenics. Their claims relate to the customer’s medical device products and sterilization cycles, and the work those employees perform at the customer’s facility, not at the Sterigenics facility.
Sterigenics and its employees did not cause any injury to the plaintiffs. The allegations asserted in this lawsuit against Sotera Health, Sterigenics and Sterigenics’ employees are baseless, and we will vigorously defend against them.
The plaintiff’s claims do not relate to Sterigenics’ state-of-the-art emissions controls at its Atlanta facility.
Sterigenics safely manages all steps of EO processing at its facility through the time the customer retrieves the customer’s product from the Sterigenics facility.